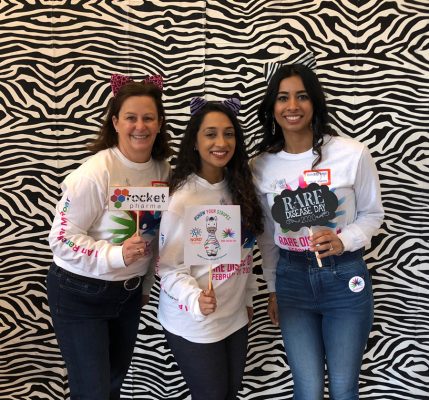RARE DISEASE DAY 2020

Rocket Pharma was proud to commemorate Rare Disease Day in New York City at “I am Rare, Hear me Roar,” an inspiring patient-centered event at the Weill Music Room in iconic Carnegie Hall. Ushering in 2020, we hope to channel the Roaring ‘20s, a decade that saw the discovery of ground-breaking medicines. Over 200 members of the rare disease and broader New York City biotech communities came together to learn firsthand the impact of rare diseases on patients and family members and how to partner in the fight to find new treatments. The event was capped with the lighting of the Empire State Building in Rare Disease Day colors to honor those affected by rare diseases and stand in solidarity with the rare disease community.
Rare Disease Day 2020 Video Presentation

Welcome
Stephanie Sirota – Chief Business Officer, RTW Investments

Introduction to Rare Disease Day Part 1
Kinnari Patel, PharmD – COO & EVP, Development, Rocket Pharma

Introduction to Rare Disease Day Part 2
Kinnari Patel, PharmD – COO & EVP, Development, Rocket Pharma

Introduction to Congresswoman Carolyn Maloney
Dr. Gayatri Rao – VP, Rocket Pharma

Remarks by Congresswoman Carolyn Maloney
U.S. Representative for New York’s 12th Congressional District

Diagnostic Journey & Impact of a Rare Disease Diagnosis Part 1
Marlene Botta
Ashlin Veselka

Diagnostic Journey & Impact of a Rare Disease Diagnosis Part 2
Marlene Botta
Ashlin Veselka

Diagnostic Journey & Impact of a Rare Disease Diagnosis Part 3
Marlene Botta
Ashlin Veselka

Diagnostic Journey & Impact of a Rare Disease Diagnosis Part 4
Marlene Botta
Ashlin Veselka

Diagnostic Journey & Impact of a Rare Disease Diagnosis Part 5
Marlene Botta
Ashlin Veselka

Book Reading from “Structured Love” Part 1
Allie M. Jones

Book Reading from “Structured Love” Part 2
Allie M. Jones
The Promise of Gene Therapy Part 1
Marley Gaskins & Tamara Hogue
Dr. Don Kohn – Professor of MIMG, Pediatrics and MMP at UCLA and Principal Investigator (LAD-I)

The Promise of Gene Therapy Part 2
Marley Gaskins & Tamara Hogue
Dr. Don Kohn – Professor of MIMG, Pediatrics and MMP at UCLA and Principal Investigator (LAD-I)

The Promise of Gene Therapy Part 3
Marley Gaskins & Tamara Hogue
Dr. Don Kohn – Professor of MIMG, Pediatrics and MMP at UCLA and Principal Investigator (LAD-I)

The Promise of Gene Therapy Part 4
Marley Gaskins & Tamara Hogue
Dr. Don Kohn – Professor of MIMG, Pediatrics and MMP at UCLA and Principal Investigator (LAD-I)

Embracing the Rare
Part 1
Jack Timperley

Embracing the Rare
Part 2
Jack Timperley

Call to Action
Part 1
Dr. John Wagner – Director, Institute for Cell, Gene and Immunotherapeutics, University of Minnesota and Scientific Advisor (Fanconi Anemia)

Call to Action
Part 2
Dr. John Wagner – Director, Institute for Cell, Gene and Immunotherapeutics, University of Minnesota and Scientific Advisor (Fanconi Anemia)

Call to Action
Part 3
Dr. John Wagner – Director, Institute for Cell, Gene and Immunotherapeutics, University of Minnesota and Scientific Advisor (Fanconi Anemia)

Closing Remarks
Dr. Roderick Wong – Chairman, Rocket Pharma Managing Partner, RTW Investments

Introduction of BioMusica and Opening of the Reception
Part 1
Dr. Gaurav Shah – CEO and President, Rocket Pharma

Introduction of BioMusica and Opening of the Reception
Part 2
Kinnari Patel, PharmD – COO & EVP, Development, Rocket Pharma