Our Science

Rocket is driven by decades of experience bringing medicines to patients who need them most. In close partnership with patient, medical and scientific communities, Rocket is harnessing cutting-edge science and innovation to correct diseases at the genetic level to enable people to live long and full lives.
Our Approach
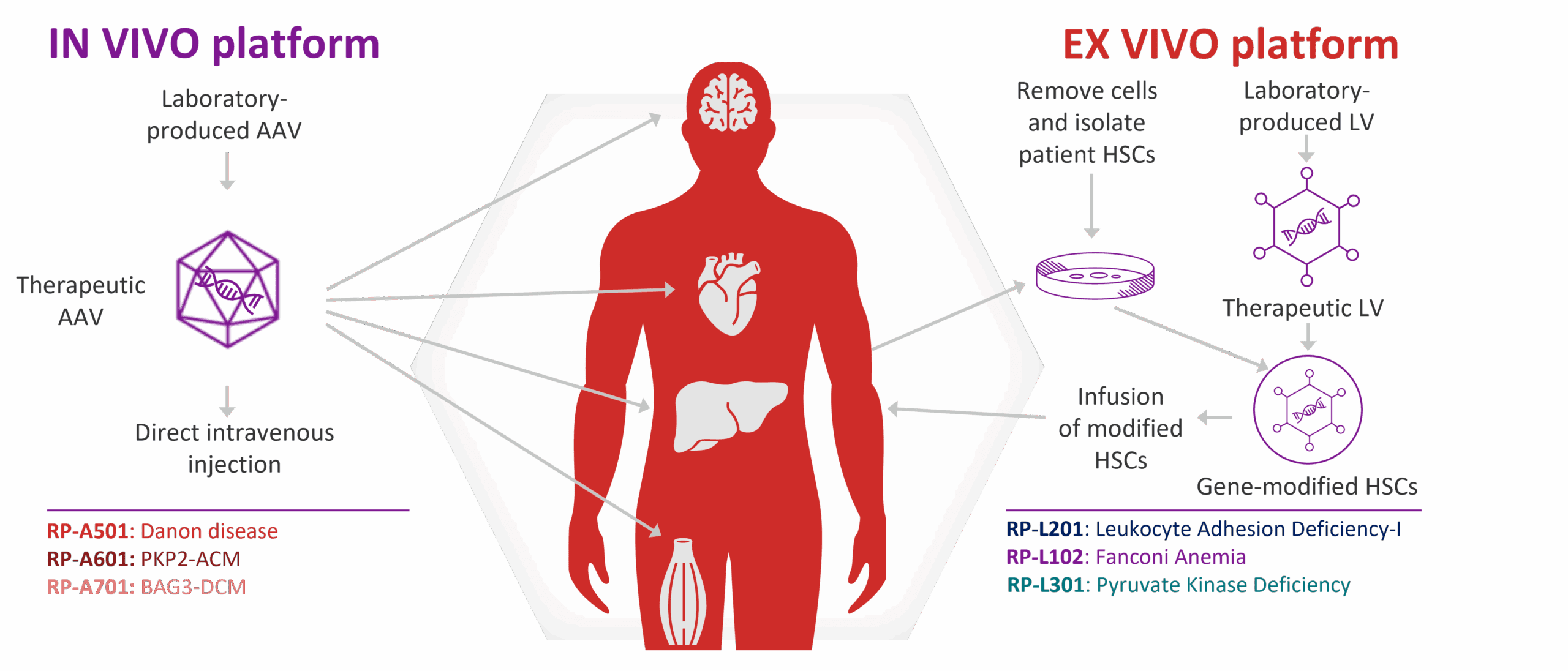
Rocket selects indications driven by a single gene in specific cell types and develops therapies that directly target the genetic mutation in the affected cells. Our innovative multi-platform approach provides us with two gene therapy platforms – in vivo adeno-associated viral (AAV) and ex vivo lentiviral (LV) technologies. Each program is intended to be transformative, enabling not only correction of the underlying defect at molecular and cellular levels, but sustained relief from debilitating and potentially life-threatening symptoms.
Collectively, our strategy allows for the creation of potentially best-in-class gene therapy candidates aimed at correcting the root cause of rare genetic disorders, spanning cardiac and hematologic indications, offering the potential for transformative and durable clinical benefits.
AAV
The AAV platform is ideal for disorders that affect the heart, liver, eye or central nervous system.
The AAV transduction process occurs in vivo (inside the body) with the Rocket team engineering each AAV construct to incorporate the corrected gene. The “therapeutic AAV” is infused directly into a patient, resulting in expression of a healthy, disease-modifying therapeutic protein in the targeted diseased cells.
Different AAV serotypes preferentially bind to specific receptors, which enables scientists to utilize specific serotypes to increase the likelihood of successful targeting of particular cell types. For example, the AAV9 and AAVrh74 serotypes have been shown to have a particular propensity for heart muscle cells.
As with the LV-platform, the ultimate goal is to allow for sufficient quantities of healthy protein to be produced by a patient’s own cells.
LV
The LV platform is ideal for modifying hematopoietic stem cells (HSCs) to address disorders affecting the bone marrow. The LV transduction process occurs ex vivo (outside the body), which enables the gene to be integrated in HSCs before the therapy is given to the patient.
The process involves collection and isolation of a patient’s HSCs, insertion of the corrected gene into the HSCs via a LV outside the body, and the infusion of modified HSCs back into the patient.
The goal is to enable sufficient quantities of a healthy, disease-modifying therapeutic protein to be manufactured by the patients’ own hematopoietic cells.
Our Pipeline
Rocket’s pipeline is comprised of first-in-class gene therapies that incorporate either AAV or LV approaches to gene therapy.

Discovery
Rocket’s Research and Development group in the Cranbury, New Jersey facility utilizes approximately 30,000 square feet of state-of-the-art laboratories for designing, optimizing and advancing candidate therapeutics to clinical stage development.Manufacturing
Rocket’s manufacturing facility in Cranbury, New Jersey recently constructed in 2022 was designed specifically for Rocket’s manufacturing needs. Approximately half of the 103,720 square foot facility is dedicated to AAV Current Good Manufacturing Practice (cGMP) manufacturing.

